
Most of the currently available vaccines against the coronavirus disease 2019 (COVID-19) are considered safe and effective, even as several variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have emerged with partial immune escape characteristics. However, the degree of protective immunity is suggested to wane over time, which has encouraged the administration of booster doses after six months.
Study: Safety and Immunogenicity of Seven COVID-19 Vaccines as A Third Dose (Booster) Following Two Doses of Chadox1 Ncov-19 Or BNT162b2 In the UK (COV-BOOST): A Blinded, Multicentre, Randomised, Controlled, Phase 2 trial. Image Credit: Wachiwit / Shutterstock.com
Background
A new Lancet preprint investigates the efficacy of this practice by using seven different COVID-19 vaccines as the third dose to boost immunity in vulnerable patients and thus reduce the impact of the virus on healthcare and the economy. In support of this, some research suggests increased immunogenicity with a heterologous prime-boost schedule compared to a homologous schedule, though this may be accompanied by increased reactogenicity.
Non-human primates and humans have been found to be protected against SARS-CoV-2, the agent that causes COVID-19, by specific antibodies formed against the virus following either natural infection or immunization. T-cells are responsible for cell-mediated immunity that prevents progression to severe disease.
The cellular adaptive immune response is key to ensuring a broadly protective response. This remains true despite the emergence of new variants with mutations in the spike protein that engages the host cell and T-cell receptors, as these mutations do not affect T-cell recognition.
Even as neutralizing antibody (nAb) levels wane, there is a measure of protection against SARS-CoV-2 variants of concern (VOCs). Both the nucleic acid and adenovirus vector vaccines produce nAbs, though the response is consistently greater with the former.
T-cell responses are similar for both types of vaccines. Heterologous regimens also elicit improved T cell responses.
About the study
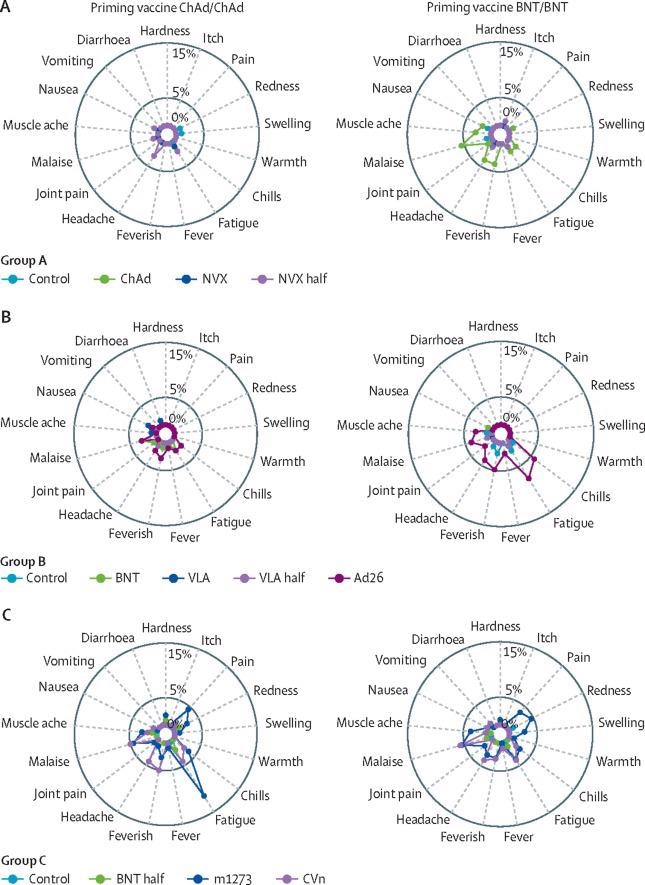
The current study reports the results of the Evaluating COVID-19 Vaccination Boosters (COV-BOOST) trial, which investigated the reactogenicity and immunogenicity of seven different COVID-19 vaccines administered as a third booster dose. The vaccines used for the prime vaccine series were either ChAdOx1 nCov-19 (Oxford–AstraZeneca; in short, ChAd) BNT162b2 (Pfizer–BioNtech or in short, BNT).
The seven vaccines used for the heterologous third-dose boost included:
Group A:
- NVX-CoV2373 (Novavax or NVX)
- A half dose of NVX
- A half-dose of ChAd
- A half-dose of quadrivalent meningococcal conjugate vaccine (MenACWY) as control
Group B:
- BNT
- VLA2001 (Valneva, or VLA)
- A half dose of VLA
- A half-dose of Ad26.COV2.S (Janssen or Ad26)
- A half-dose of MenACWY
Group C:
- mRNA1273 (Moderna, or m1273),
- CVnCov (CureVac or CVn)
- A half dose of BNT
- A half-dose of MenACWY
The researchers not only examined the effect of using different vaccines for the boost but also assessed three vaccines that were given at full doses and also at a half dose. These different doses were administered to determine whether reducing the dosage with the third dose would both reduce reactogenicity and preserve immunogenicity, while simultaneously stretching the vaccine supply.
All 2,878 participants receiving a third dose vaccine were over the age of 30 and had been fully vaccinated 70 or more days prior in the case of ChAD, or 84 days ago with BNT. The median age was 53 years and 51 years for younger patients in the ChAD and BNT groups, respectively, whereas older patients had a median age of 76 years and 78 years, respectively. Most patients were white and about half in each group were female.
Study findings
In the individuals who had completed the ChAd prime series, antibody titers, as assessed by the geometric mean ratios (GMRs) of spike immunoglobulin G (IgG), covered a wide range from 1.8 to 32.3 for the half-dose VLA and the m1273 vaccines, respectively. In this set, wild-type cellular responses showed GMRs that were somewhat lower for ChAd and down to a tenth in the m1273 group.
Neutralizing responses were correlated with anti-spike IgG GMRs. Except for ChAd and VLA at half- or full-dosage, all vaccines induced T-cell responses.
In the BNT prime series group, the GMRs for total antibodies ranged from 1.3 to 11.5 for the two vaccine boosters above, respectively. The wild-type cellular responses were again lower, at GMRs of 1 and 4.7 for the two booster vaccines.
T-cell responses were lower in the BNT prime series group when exposed to a ChAd booster as compared to the ChAd prime series with a third-dose ChAd booster. Neutralization of the SARS-CoV-2 Delta variant was reduced as compared to the wild-type strain for all vaccine boosters in both groups to a similar extent. However, T-cell responses were similar for Delta, Beta, and wild-type strains.
The third-dose boosters showed similar results in adults aged 30 years and above. The most common adverse events were fatigue and pain, while those aged 30-69 years reported these more frequently. Serious adverse reactions were not more common in the treatment group as compared to controls and were equally distributed between the vaccines.
Implications
The study shows that antibody responses and virus-neutralizing responses were alike boosted after a booster dose with any of the seven vaccines in patients who had received two doses of the ChAd vaccine initially, and almost all showed a similar response after two doses of the BNT vaccine. The vaccines were safe and well-tolerated.
Either a homologous or heterologous third dose vaccine boosts immunity with all the vaccines tested, irrespective of the prime series vaccine used. The only exception was VLA.
Again, both ChAd and BNT have produced excellent real-world protection against hospitalization and death, even at six months after full vaccination, even though the spike IgG levels achieved with the ChAd vaccine are much lower than those achieved by the BNT vaccine. The interval between doses and the T-cell response may account for this lack of correlation.
T-cell responses are not boosted further with a ChAd booster after a homologous prime series. The VLA also does not produce a significant cellular response to the spike antigen as compared to the control after either priming regimen.
The data “could suggest that currently approved mRNA vaccines are formulated at doses above the minimum needed for a third (booster) dose.”
Lowering the dosage for the third dose could also ensure that inflammation and myocarditis rates are minimized after messenger ribonucleic acid (mRNA) vaccine third doses. In fact, m1273 is now approved for use as a half-dose for a homologous third-dose booster.
Booster-dose vaccines can therefore be chosen from an array of heterologous vaccines based on the differential responses in antibody and cellular immune response, along with vaccine availability.
Journal reference:
- Munro, A. P. S., Janani, L., Cornelius, V., et al. (2021). Safety and Immunogenicity of Seven COVID-19 Vaccines as A Third Dose (Booster) Following Two Doses of Chadox1 Ncov-19 Or BNT162b2 In the UK (COV-BOOST): A Blinded, Multicentre, Randomised, Controlled, Phase 2 trial. The Lancet. doi:10.1016/S0140-6736(21)02717-3. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02717-3/fulltext.